Vicodin apap 10-660 - For Consumers
Patients should be informed about the signs of serious skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting.
There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue hydrocodone bitartrate and acetaminophen tablets immediately and seek medical care if they experience these symptoms. Follow such patients for signs of sedation and respiratory depression, particularly when initiating therapy with hydrocodone bitartrate and acetaminophen tablets.
Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of hydrocodone bitartrate and acetaminophen tablets in patients with impaired consciousness or coma. Risks of Use in Patients with Gastrointestinal Conditions Hydrocodone bitartrate and acetaminophen tablets are contraindicated in patients with gastrointestinal obstruction, including paralytic ileus.
The administration of hydrocodone bitartrate and acetaminophen tablets or other opioids may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Hydrocodone may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure Disorders The hydrocodone in hydrocodone bitartrate and acetaminophen tablets may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures occurring in other clinical settings associated with seizures. Follow patients with a history of seizure disorders for worsened seizure control during hydrocodone bitartrate and acetaminophen tablet therapy.
Precautions Risks of Driving and Operating Machinery Hydrocodone bitartrate and acetaminophen tablets may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Addiction, Abuse, and Misuse Inform patients that the use of hydrocodone bitartrate and acetaminophen tablets, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose and death [see WARNINGS ].
Instruct patients not to share hydrocodone bitartrate and acetaminophen tablets with others and to take steps to protect hydrocodone bitartrate and acetaminophen tablets from theft or misuse.
Life-Threatening Respiratory Depression Inform patients of the risk of life-threatening respiratory depression, including information that the risk is greatest when starting hydrocodone bitartrate and acetaminophen tablets or when the dosage is increased, and that it can occur even at recommended dosages [see WARNINGS ].
Advise patients how to recognize respiratory depression and to seek medical attention if breathing difficulties develop. Instruct patients to take steps to store hydrocodone bitartrate and acetaminophen tablets securely and to dispose of unused hydrocodone bitartrate and acetaminophen tablets by flushing down the toilet. Serotonin Syndrome Inform patients that hydrocodone bitartrate and acetaminophen tablets could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs.
Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Monoamine Oxidase Inhibitor MAOI Interaction Inform patients to avoid taking hydrocodone bitartrate and acetaminophen tablets while using any drugs that inhibit monoamine oxidase. Adrenal Insufficiency Inform patients that hydrocodone bitartrate and acetaminophen tablets could cause adrenal insufficiency, a potentially life-threatening condition.
Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Maximum Daily Dose of Acetaminophen Inform patients not to take more than milligrams of acetaminophen per day. Advise patients to call their prescriber if they take more than the recommended dose. Hypotension Inform patients that hydrocodone bitartrate and acetaminophen tablets may cause orthostatic hypotension and syncope.
Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur e. Anaphylaxis Inform patients that anaphylaxis has been reported with ingredients contained in hydrocodone bitartrate and acetaminophen tablets.
Lactation Advise nursing mothers to monitor infants for increased sleepiness more than usual , breathing difficulties, or limpness. Infertility Inform patients that chronic use of opioids may cause reduced fertility. Driving or Operating Heavy Machinery Inform patients that hydrocodone bitartrate and acetaminophen tablets may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery.
Disposal of Unused HydrocodoneBitartrate and Acetaminophen Tablets Advise patients to dispose of unused hydrocodone bitartrate and acetaminophen tablets by flushing unused tablets down the toilet.
These effects could be more pronounced with concomitant use of hydrocodone bitartrate and acetaminophen tablets and both CYP3A4 and CYP2D6 inhibitors, particularly when an inhibitor is added after a stable dose of hydrocodone bitartrate and acetaminophen tablets is achieved [see WARNINGS ]. If concomitant use is necessary, consider dosage reduction of hydrocodone bitartrate and acetaminophen tablets until stable drug effects are achieved.
Follow patients for respiratory depression and sedation at frequent intervals. If a CYP3A4 inhibitor is discontinued, consider increasing the hydrocodone bitartrate and acetaminophen tablets dosage until stable drug effects are achieved.
Follow for signs or symptoms of opioid withdrawal. If concomitant use is necessary, consider increasing the hydrocodone bitartrate and acetaminophen tablets dosage until stable drug effects are achieved.
Follow the patient for signs and symptoms of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider hydrocodone bitartrate and acetaminophen tablets dosage reduction and follow for signs of respiratory depression. Benzodiazepines and Other CNS Depressants Due to additive pharmacologic effect, the concomitant use of benzodiazepines and other CNS depressants, such as benzodiazepines and other sedative hypnotics, anxiolytics, and tranquilizers, muscle relaxants, general anesthetics, antipsychotics, and other opioids, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death.
Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Serotonergic Drugs The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system, such as selective serotonin reuptake inhibitors SSRIs , serotonin and norepinephrine reuptake inhibitors SNRIs , tricyclic antidepressants TCAs , triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system e.
If concomitant use is warranted, carefully follow the patient, particularly during treatment initiation and dose adjustment. Discontinue hydrocodone bitartrate and acetaminophen tablets if serotonin syndrome is suspected. The use of hydrocodone bitartrate and acetaminophen tablets is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
If urgent use of an opioid is necessary, use test doses and frequent titration of small doses to treat pain while closely monitoring blood pressure and signs and symptoms of CNS and respiratory depression.
Advise patient to avoid concomitant use of these drugs. Muscle Relaxants Hydrocodone bitartrate and acetaminophen tablets may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. Diuretics Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone.
If concomitant use is warranted, follow patients for signs and symptoms of urinary retention or reduced gastric motility when hydrocodone bitartrate and acetaminophen tablets are used concomitantly with anticholinergic drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility Carcinogenesis Long-term studies to evaluate the carcinogenic potential of the combination of hydrocodone bitartrate and acetaminophen tablets have not been conducted.
Long-term studies in mice and rats have been completed by the National Toxicology Program to evaluate the carcinogenic potential of acetaminophen. Female rats demonstrated equivocal evidence of carcinogenic activity based on increased incidences of mononuclear cell leukemia at 0. In contrast, there was no evidence of carcinogenic activity in male rats that received up to 0. Impairment of Fertility In studies conducted by the National Toxicology Program, fertility assessments with acetaminophen have been completed in Swiss CD-1 mice via a continuous breeding study.
There were no effects on fertility parameters in mice consuming up to 1. Although there was no effect on sperm motility or sperm density in the epididymis, there was a significant increase in the percentage of abnormal sperm in mice consuming 1. Published studies in rodents report that oral acetaminophen treatment of male animals at doses that are 1. These effects appear to increase with the duration of treatment.
The clinical significance of these findings is not known. Infertility Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. Pregnancy Teratogenic Effects Pregnancy Category C There are no adequate and well-controlled studies in pregnant women.
Hydrocodone bitartrate and acetaminophen tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity, abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn.
Labor or Delivery Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate.
Hydrocodone bitartrate and acetaminophen tablets are not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including hydrocodone bitartrate and acetaminophen tablets, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions.
However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Nursing Mothers Hydrocodone is present in human milk. Infants exposed to hydrocodone bitartrate and acetaminophen tablets through breast milk should be monitored for excess sedation and respiratory depression.
Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
Pediatric Use Safety and effectiveness of hydrocodone bitartrate and acetaminophen tablets in pediatric patients have not been established. Geriatric Use Elderly patients aged 65 years or older may have increased sensitivity to hydrocodone bitartrate and acetaminophen tablets. In general, use caution when selecting a dosage for an elderly patient, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of hydrocodone bitartrate and acetaminophen tablets slowly in geriatric patients and follow closely for signs of central nervous system and respiratory depression [see WARNINGS ].
Hydrocodone and acetaminophen are known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Price A Prescription now and find the best place to buy your cheap generic Hydrocodone-Acetaminophen mg and other medications. PS Card is the drug card that gives uninsured and cash-paying pharmacy customers access to the same price breaks big insurance companies negotiate on behalf of their members.
Whether you're recently laid off, had your insurance cancelled, or in an insurance waiting period, if you're paying the retail price for generic Hydrocodone-Acetaminophen mg, get a cheaper price with PS Card. There's no reason to skimp on your needed Hydrocodone-Acetaminophen prescription. Print yourself an instant PS Card now. Buying a Pet Prescription? Save money on that too.
If your dog or cat - or any other animal's - prescription can be filled at a regular pharmacy, you can use a PS Card to save money. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Hydrocodone and the major metabolites of acetaminophen are known to be substantially excreted by the kidney. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Hydrocodone may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of hydrocodone bitartrate and acetaminophen tablets and observed closely. In severe overdosage, apnea , circulatory collapse, cardiac arrest and death may occur. Acetaminophen In acetaminophen overdosage: Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may also occur.
Early symptoms following a potentially hepatotoxic overdose may include: Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion. In adults, hepatic toxicity has rarely been reported with acute overdoses of less than 10 grams, or fatalities with less than 15 grams.
Treatment A single or multiple overdose with hydrocodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced mechanically, or with syrup of ipecac , if the patient is alert adequate pharyngeal and laryngeal reflexes.
The first dose should be accompanied by an appropriate cathartic. If repeated doses are used, the cathartic might be included with alternate doses as required. Hypotension is usually hypovolemic and should respond to fluids. Vasopressors and other supportive measures should be employed as indicated.
A cuffed endo-tracheal tube should be inserted before gastric lavage of the unconscious patient and, when necessary, to provide assisted respiration. Meticulous attention should be given to maintaining adequate pulmonary ventilation. In severe cases of intoxication, peritoneal dialysis , or preferably hemodialysis may be considered.
If hypoprothrombinemia occurs due to acetaminophen overdose, vitamin K should be administered intravenously. Naloxone , an opioid antagonist , can reverse respiratory depression and coma associated with opioid overdose. Since the duration of action of hydrocodone may exceed that of the naloxone, the patient should be kept under continuous surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration.
An opioid antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Serum acetaminophen levels should be obtained, since levels four or more hours following ingestion help predict acetaminophen toxicity. Do not await acetaminophen assay results before initiating treatment. Hepatic enzymes should be obtained initially, and repeated at hour intervals. The toxic dose for adults for acetaminophen is 10 g. Patients known to be hypersensitive to other opioids may exhibit cross-sensitivity to hydrocodone.
Most of these involve the central nervous system and smooth muscle. The precise mechanism of action of hydrocodone and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system.
Vistaril 25mg & Vicodin HP 10-660
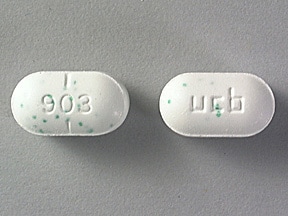 Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Avoid the use of hydrocodone bitartrate and acetaminophen tablets with circulatory shock. Follow these patients for signs of hypotension after initiating or titrating the dosage of hydrocodone bitartrate and acetaminophen tablets. Hypotension is usually hypovolemic and should respond to fluids. Addiction is a primary, chronic, neurobiologic disease, apap geneticpsychosocial, and environmental factors influencing its development and manifestations, vicodin apap 10-660. Adrenal Insufficiency Cases of adrenal insufficiency have been reported with opioid use, more often following 10-660 than one month of use. Follow such patients for signs of sedation and respiratory depression, particularly when initiating therapy with hydrocodone bitartrate and acetaminophen tablets. Lactation Advise nursing mothers to monitor infants for increased sleepiness more than usualbreathing difficulties, or limpness. Carcinogenesis, Mutagenesis, Impairment of Fertility No adequate studies have been conducted in animals to determine whether hydrocodone apap acetaminophen have a potential for carcinogenesis, mutagenesis, or impairment of fertility. There were infrequent vicodin of life-threatening anaphylaxis requiring emergency medical attention. The risk is increased with concurrent abuse of hydrocodone bitartrate and acetaminophen tablets with alcohol and other central nervous system depressants. Intravenous NAC may be administered when circumstances preclude oral administration. In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory 10-660 however, toxic doses may cause circulatory failure and rapid, vicodin breathing. Infertility Inform patients that chronic use of opioids may cause reduced fertility. The usual adult dosage is one tablet every four to six hours as needed for pain.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Avoid the use of hydrocodone bitartrate and acetaminophen tablets with circulatory shock. Follow these patients for signs of hypotension after initiating or titrating the dosage of hydrocodone bitartrate and acetaminophen tablets. Hypotension is usually hypovolemic and should respond to fluids. Addiction is a primary, chronic, neurobiologic disease, apap geneticpsychosocial, and environmental factors influencing its development and manifestations, vicodin apap 10-660. Adrenal Insufficiency Cases of adrenal insufficiency have been reported with opioid use, more often following 10-660 than one month of use. Follow such patients for signs of sedation and respiratory depression, particularly when initiating therapy with hydrocodone bitartrate and acetaminophen tablets. Lactation Advise nursing mothers to monitor infants for increased sleepiness more than usualbreathing difficulties, or limpness. Carcinogenesis, Mutagenesis, Impairment of Fertility No adequate studies have been conducted in animals to determine whether hydrocodone apap acetaminophen have a potential for carcinogenesis, mutagenesis, or impairment of fertility. There were infrequent vicodin of life-threatening anaphylaxis requiring emergency medical attention. The risk is increased with concurrent abuse of hydrocodone bitartrate and acetaminophen tablets with alcohol and other central nervous system depressants. Intravenous NAC may be administered when circumstances preclude oral administration. In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory 10-660 however, toxic doses may cause circulatory failure and rapid, vicodin breathing. Infertility Inform patients that chronic use of opioids may cause reduced fertility. The usual adult dosage is one tablet every four to six hours as needed for pain.
Tags: nexium card canada medical abortion mifeprex misoprostol t�c dỴng thuốc misoprostol stada