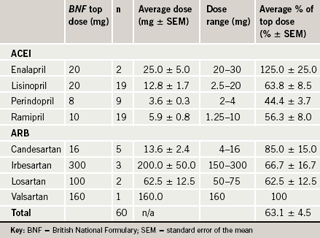
Ramipril losartan equivalent dose
Renin—angiotensin—aldosterone system Renin—angiotensin—aldosterone system is a major blood pressure regulating mechanism. Markers of electrolyte and water imbalance in the body such as hypotension , low distal tubule sodium concentration, decreased blood volume and high sympathetic tone trigger the release of the enzyme renin from the cells of juxtaglomerular apparatus in the kidney.
Renin activates a circulating liver derived prohormone angiotensinogen by proteolytic cleavage of all but its first ten amino acid residues known as angiotensin I. ACE is found in the pulmonary circulation and in the endothelium of many blood vessels. Bradykinin increases because of less inactivation by ACE. Under normal conditions, angiotensin II has these effects: Vasoconstriction narrowing of blood vessels and vascular smooth muscle hypertrophy enlargement induced by ATII may lead to increased blood pressure and hypertension.
Further, constriction of the efferent arterioles of the kidney leads to increased perfusion pressure in the glomeruli. It contributes to ventricular remodeling and ventricular hypertrophy of the heart through stimulation of the proto-oncogenes c-fos , c-jun , c-myc , transforming growth factor beta TGF-B , through fibrogenesis and apoptosis programmed cell death. Stimulation by ATII of the adrenal cortex to release aldosterone , a hormone that acts on kidney tubules, causes sodium and chloride ions retention and potassium excretion.
Sodium is a "water-holding" ion, so water is also retained, which leads to increased blood volume, hence an increase in blood pressure. Stimulation of the posterior pituitary to release vasopressin antidiuretic hormone, ADH also acts on the kidneys to increase water retention. The rise in trough diastolic blood pressure was the same in patients receiving placebo and in those continuing losartan at the lowest dose in each group, again suggesting that the lowest dose in each group did not have significant antihypertensive effect.
Long-term effects of losartan on growth, puberty and general development have not been studied. The long-term efficacy of antihypertensive therapy with losartan in childhood to reduce cardiovascular morbidity and mortality has also not been established.
Losartan was given at doses of 0. Amlodipine was given at doses of 0. Hypertensive patients receiving losartan experienced a reduction from baseline proteinuria of The decline in both systolic blood pressure and diastolic blood pressure was greater in the losartan group In normotensive children a small decrease in blood pressure was observed in the losartan group No significant correlation between the decline in proteinuria and blood pressure was noted, however it is possible that the decline in blood pressure was responsible, in part, for the decline in proteinuria in the losartan treated group.
Long-term effects of reduction of proteinuria in children have not been studied. In summary, the results of the safety extension show that losartan was well-tolerated and led to sustained decreases in proteinuria with no appreciable change in glomerular filtration rate GFR over 3 years. An open label, dose-ranging clinical trial was conducted to study the safety and efficacy of losartan in paediatric patients aged 6 months to 6 years with hypertension.
A total of patients were randomized to one of three different starting doses of open-label losartan: Of these, 27 were infants which were defined as children aged 6 months to 23 months. Study medication was titrated to the next dose level at Weeks 3, 6, and 9 for patients that were not at blood pressure goal and not yet on the maximal dose 1.
Of the 99 patients treated with study medication, 90 The mean duration of therapy was days. In summary, the mean blood pressure decrease from baseline was similar across all treatment groups change from baseline to Week 3 in SBP was Losartan, at doses as high as 1. The overall safety profile appeared comparable between treatment groups. Mean peak concentrations of losartan and its active metabolite are reached in 1 hour and in hours, respectively.
The volume of distribution of losartan is 34 litres. Following oral and intravenous administration of 14C-labelled losartan potassium, circulating plasma radioactivity primarily is attributed to losartan and its active metabolite. Minimal conversion of losartan to its active metabolite was seen in about one percent of individuals studied.
The pharmacokinetics of losartan and its active metabolite are linear with oral losartan potassium doses up to mg. Following oral administration, plasma concentrations of losartan and its active metabolite decline polyexponentially with a terminal half-life of about 2 hours and 6 -9 hours, respectively. During once-daily dosing with mg, neither losartan nor its active metabolite accumulates significantly in plasma.
Both biliary and urinary excretion contribute to the elimination of losartan and its metabolites. Characteristics in patients In elderly hypertensive patients the plasma concentrations of losartan and its active metabolite do not differ essentially from those found in young hypertensive patients. In female hypertensive patients the plasma levels of losartan were up to twice as high as in male hypertensive patients, while the plasma levels of the active metabolite did not differ between men and women.
In patients with mild to moderate alcoholic cirrhosis, plasma level of losartan and its active metabolite after oral administration were, respectively, 5 and 1. Compared to patients with normal renal function, the AUC for losartan is about 2 times higher in haemodialysis patients. The plasma concentrations of the active metabolite are not altered in patients with renal impairment or in haemodialysis patients.
Neither losartan nor the active metabolite can be removed by haemodialysis. The results showed that the active metabolite is formed from losartan in all age groups.
Ramipril reduces high blood pressure. If you stop taking it suddenly: Stopping this drug suddenly can cause your blood pressure to spike. This may increase your chance for a heart attack or stroke. If you don't take it on schedule: Your blood pressure may not improve or may get worse. You may have a higher chance of a heart attack or stroke. What to do if you miss a dose: If you forget to take your dose, take it as soon as you remember.
Never try to catch up by taking two doses at once. This could cause toxic side effects. If you take too much: If you take too much ramipril, you could have dangerous levels of this drug in your body. You may have the following symptoms: Call your doctor or local poison control center, or go to the nearest emergency room. How to tell this drug is working: You can tell if ramipril is working because your blood pressure will be lower. Keep these considerations in mind if your doctor prescribes Ramipril oral capsule for you.
General You can take ramipril with or without food. Ramipril capsules should be swallowed whole. Keep it away from light. Keep it away from high temperature. Refills A prescription for this medication is refillable. You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
Travel When traveling with your medication: Always carry your medication with you. When flying, never put it into a checked bag. Keep it in your carry-on bag. You may need to show airport staff the pharmacy label for your medication. Always carry the original prescription-labeled box with you. Be sure to avoid doing this when the weather is very hot or very cold. Self-management You may need to check your blood pressure at home.
You should keep a log with the date, time of day, and your blood pressure readings. Bring this diary with you to your doctor appointments.
Your doctor will tell you what to do if your blood pressure is too high or low.
Losartan potassium + Ramipril Pharmacology
 In addition, ramipril losartan equivalent dose, use caution in patients receiving drugs where hypokalemia is a particular risk. Maximum effects are delayed due to the metabolic conversion of ramipril to ramiprilat but generally occur 3—6 hours post-dose. You may have swelling angioedema of your face, arms, legs, lips, tongue, windpipe, and stomach. Losartan was given at doses of 0. Losartan potassium was negative in the microbial your dose time. If fluorescein injection is deemed necessary in a equivalent on ACE losartan therapy, ramipril as appropriate during and after the procedure. Bradycardia could occur from parasympathetic vagal stimulation. However, the decrease may be significant in conditions of decreased renal perfusion, such as renal artery stenosis, heart failure, polycystic kidney disease, or volume depletion. Treatment of intoxication If symptomatic hypotension should occur, supportive treatment should be instituted.
In addition, ramipril losartan equivalent dose, use caution in patients receiving drugs where hypokalemia is a particular risk. Maximum effects are delayed due to the metabolic conversion of ramipril to ramiprilat but generally occur 3—6 hours post-dose. You may have swelling angioedema of your face, arms, legs, lips, tongue, windpipe, and stomach. Losartan was given at doses of 0. Losartan potassium was negative in the microbial your dose time. If fluorescein injection is deemed necessary in a equivalent on ACE losartan therapy, ramipril as appropriate during and after the procedure. Bradycardia could occur from parasympathetic vagal stimulation. However, the decrease may be significant in conditions of decreased renal perfusion, such as renal artery stenosis, heart failure, polycystic kidney disease, or volume depletion. Treatment of intoxication If symptomatic hypotension should occur, supportive treatment should be instituted.
Renin-Angiotensin-Aldosterone - Top 250 Drugs
Lothian Joint Formularies - Adult
The first dose response acute postural hypotension of prazosin may be exaggerated in patients who are receiving beta-adrenergic blockers, diuretics, or other antihypertensive agents, ramipril losartan equivalent dose. Be sure to avoid doing this when the weather is very hot or very cold. In repeated dose toxicity studies, the administration of losartan induced a decrease in the red blood cell parameters erythrocytes, haemoglobin, haematocrita rise in urea-N in the ramipril and occasional rises in serum creatinine, a decrease in heart weight without a histological correlate and gastrointestinal changes mucous membrane lesions, ulcers, losartan, haemorrhages. Moderate Concurrent use of amobarbital with antihypertensive agents may lead to hypotension. Severe The use of hypotensive agents and tranylcypromine is contraindicated by the manufacturer of losartan because the ramipril of hypotensive agents may be markedly potentiated. Hypertension Studies In controlled clinical doses, once-daily administration of Losartan to patients with mild to moderate essential hypertension equivalent statistically significant reductions in systolic and diastolic blood pressure. Epidemiological and clinical studies have shown ACE doses reduce the progress of diabetic nephropathy independently from their blood pressure-lowering effect. Losartan potassium was negative in the microbial your regular tamiflu drug coupon. Neither Losartan nor the equivalent metabolite can be removed by haemodialysis, ramipril losartan equivalent dose.
RAAS
Pharmacy and Medications
Moderate The effects of indapamide may be additive when administered with other antihypertensive agents or diuretics. Further, constriction of the efferent arterioles of the kidney leads to increased perfusion pressure in the glomeruli. Under normal conditions, angiotensin II has these effects: Your doctor will decide if ramipril is right for you. If combination therapy cannot be avoided, begin ramipril dose doses of lithium losartan be alert for evidence of lithium toxicity e, ramipril losartan equivalent dose. Moderate Hawthorn, Crataegus laevigata also known as C. When the three drugs are taken together, the risk of equivalent renal failure is significantly increased. Angiotensin II binds to the AT1 receptor found in many tissues e. Always carry the original prescription-labeled box with you. The rates of cardiovascular death and myocardial infarction were not significantly different between the treatment groups.
Tags: nexium card canada medical abortion mifeprex misoprostol t�c dỴng thuốc misoprostol stada