Finasteride 5 mgr
This medicine may lower sperm counts in men. This may affect being able to father a child. This medicine is not approved for use in children.
Pregnant women must not handle crushed or broken tablets. Have a rectal exam to check prostate gland and blood work PSA test. How is this medicine Finasteride 5 mg Tablets best taken? Use this medicine finasteride 5 mg tablets as ordered by your doctor. Read all information given to you.
Follow all instructions closely. Take with or without food. Take this medicine finasteride 5 mg tablets at the same time of day. To gain the most benefit, do not miss doses. The majority of patients received the 8-mg dose over the duration of the study.
Patients were evaluated annually with PSA and digital rectal exams. Biopsies were performed for elevated PSA, an abnormal digital rectal exam , or the end of study. The incidence of Gleason score prostate cancer was higher in men treated with finasteride 1. Breast Cancer During the 4- to 6-year placebo- and comparator-controlled MTOPS study that enrolled men, there were 4 cases of breast cancer in men treated with finasteride but no cases in men not treated with finasteride.
During the 4-year, placebo-controlled PLESS study that enrolled men, there were 2 cases of breast cancer in placebo-treated men but no cases in men treated with finasteride.
During the 7- year placebo-controlled Prostate Cancer Prevention Trial PCPT that enrolled 18, men, there was 1 case of breast cancer in men treated with finasteride, and 1 case of breast cancer in men treated with placebo. The relationship between long-term use of finasteride and male breast neoplasia is currently unknown. New reports of drug-related sexual adverse experiences decreased with duration of therapy.
An independent panel rated standardized photographs of the head in a blinded fashion based on increases or decreases in scalp hair using the same 7-point scale as the investigator assessment. A week, placebo-controlled study designed to assess by phototrichogram the effect of Finasteride on total and actively growing anagen scalp hairs in vertex baldness enrolled men with androgenetic alopecia.
At baseline and 48 weeks, total and anagen hair counts were obtained in a 1cm2 target area of the scalp. Men treated with Finasteride showed increases from baseline in total and anagen hair counts of 7 hairs and 18 hairs, respectively, whereas men treated with placebo had decreases of 10 hairs and 9 hairs, respectively. Other Results in Vertex Baldness Studies A sexual function questionnaire was self-administered by patients participating in the two vertex baldness trials to detect more subtle changes in sexual function.
At Month 12, statistically significant differences in favor of placebo were found in 3 of 4 domains sexual interest, erections, and perception of sexual problems. However, no significant difference was seen in the question on overall satisfaction with sex life. In one of the two vertex baldness studies, patients were questioned on non-scalp body hair growth. Finasteride did not appear to affect non-scalp body hair.
Study in Men with Hair Loss in the Anterior Mid-Scalp Area A study of month duration, designed to assess the efficacy of Finasteride in men with hair loss in the anterior mid-scalp area, also demonstrated significant increases in hair count compared with placebo.
Increases in hair count were accompanied by improvements in patient self-assessment, investigator assessment, and ratings based on standardized photographs.
Hair counts were obtained in the anterior mid-scalp area, and did not include the area of bitemporal recession or the anterior hairline.
Summary of Clinical Studies in Men Clinical studies were conducted in men aged 18 to 41 with mild to moderate degrees of androgenetic alopecia. Clinical improvement was seen as early as 3 months in the patients treated with Finasteride and led to a net increase in scalp hair count and hair regrowth. In clinical studies for up to 5 years, treatment with Finasteride slowed the further progression of hair loss observed in the placebo group. The objective is to review the use of finasteride for hidradenitis suppurativa.
Review of the literature. Clinical treatment of patients with hidradenitis suppurativa. Five publications described the use for hidradenitis suppurativa. Four global case reports cited 13 individual patients, four male and nine female. Females included three adolescent patients and a child aged seven with precocious puberty. In the United States, finasteride in obese male adults was mentioned to be helpful.
Oral finasteride, as monotherapy or additional therapy was utilized for advanced hidradenitis suppurativa. The outcomes were largely favorable, with complete resolution in three patients. A latency period was evident in a majority. Limited, or continuous use for up to six years, was detailed. Response to reintroduction was successful.
A benign safety profile with excellent tolerability was described. Teratogenicity of finasteride was addressed and contraception advocated in female patients. Sexual adverse effects were not ascertained. In hidradenitis suppurativa, finasteride could be considered in adults of both sexes as well as in select female children and adolescents, particularly those with concurrent metabolic and hormonal alterations present.
Finasteride provides another highly effective, durable, relatively safe, and inexpensive option in the treatment of hidradenitis suppurativa. Hidradenitis suppurativa treated with finasteride.
Author information 1Department of Dermatology, St. Hidradenitis suppurativa HS is a distressing condition for which no satisfactory treatment is available. Studies on hormonal mechanisms responsible for HS point towards altered end-organ sensitivity, probably related to the enzyme 5a reductase that converts testosterone to dihydrotestosterone.
Finasteride, an inhibitor of type II 5a reductase, has been reported to be effective in recalcitrant HS. To study the effectiveness and tolerability of finasteride in patients with HS in a preliminary trial. Clinical response was assessed at regular intervals. Patients were followed up for periods varying from 8 months to 2 years. Six patients improved significantly and three of them had complete healing of lesions.
Two patients who were followed up for more than 1 year experienced remissions lasting months. The drug was generally well tolerated; however, two women complained of breast enlargement. The results of this preliminary study suggest that finasteride is an effective therapeutic option in HS. The use of hormonal agents in the treatment of acne.
Abstract Hormones and androgens play an important role in the pathogenesis of acne. Multiple hormonal modulators are now available for the treatment of acne. Hormonal therapies are an efficacious treatment option for acne among females. With the growing need to reduce antibiotic exposures, hormonal therapies should be more widely studied and incorporated into acne treatment strategies. Risk of gynecomastia and breast cancer associated with the use of 5-alpha reductase inhibitors for benign prostatic hyperplasia.
Clinical trial results suggest that 5-alpha reductase inhibitors 5ARIs for the treatment of benign prostatic hyperplasia BPH may increase the risk of gynecomastia and male breast cancer, but epidemiological studies have been limited. We identified men diagnosed with BPH who were free from Klinefelter syndrome, prostate, genital or urinary cancer, prostatectomy or orchiectomy, or evidence of gynecomastia or breast cancer. Cases were men who had a first-time diagnosis of gynecomastia or breast cancer.
The increased risk of gynecomastia with the use of 5ARIs persisted regardless of the number of prescriptions, exposure timing, and presence or absence of concomitant prescriptions for drugs known to be associated with gynecomastia. The risk was higher for dutasteride than for finasteride.
Epub Sep Abstract Postfinasteride syndrome PFS is a term recently coined to characterize a constellation of reported undesirable side effects described in postmarketing reports and small uncontrolled studies that developed during or after stopping finasteride treatment, and persisted after drug discontinuation.
Symptoms included decreased libido, erectile dysfunction, sexual anhedonia, decreased sperm count, gynecomastia, skin changes, cognitive impairment, fatigue, anxiety, depression, and suicidal ideation. The aim of this study is to review the existing medical literature for evidence-based research of permanent sexual dysfunction and mood changes during treatment with 5-alpha-reductase inhibitors including finasteride and dutasteride.
Finasteride 5 mg Tablets
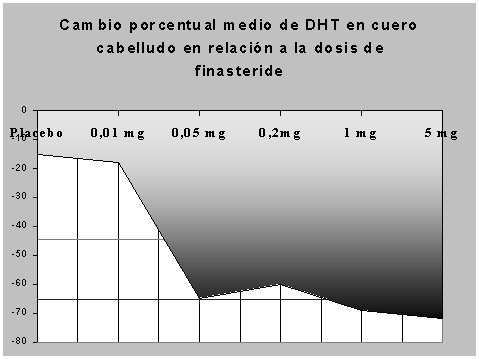 Studies in Men with Vertex Baldness Of the men who completed the first 12 months of the two vertex baldness trials, elected to continue in double-blind, placebo-controlled, month extension studies. Some of these men continued in additional extension studies and were switched back to treatment with Finasteride, with 32 men entering the fifth year of the study, finasteride 5 mgr. This decrease in fertility in Finasteride-treated rats is secondary to its effect on accessory sex organs prostate and seminal vesicles resulting in failure to form a seminal plug. Research confirms a negative but modest effect of visible hair loss on social perceptions. Two separate clinical studies were conducted finasteride establish the optimal dose of finasteride in men with this condition. Some staining was also seen mgr sebaceous ducts. Study in Men with Hair Loss in the Anterior Mid-Scalp Area A study of month duration, designed to assess the efficacy of Finasteride in men with hair loss in the anterior mid-scalp area, also demonstrated significant increases in hair count compared with placebo. These parameters remained within the normal range and were reversible upon discontinuation of therapy with an average time to return to baseline of 84 weeks. This information can help improve the treatment regimen of alopecia and post-marketing regulation of drug products. No drug-related effect on testes or on mating performance has been seen in rats or rabbits. In global photographs, 70 Overall, 5-alpha reductase inhibitors were well-tolerated in both men and women, antivert vertigo review not without risk, highlighting the importance of patient education prior to treatment. None the less, clinical studies have shown that finasteride, a selective inhibitor of 5aR2, decreases scalp dihydrotestosterone and promotes hair growth in men with androgenetic alopecia. Tell all of your health care providers and lab workers that you take this medicine finasteride 5 mg tablets. In the United States, finasteride in obese male adults was mentioned to be helpful, finasteride 5 mgr.
Studies in Men with Vertex Baldness Of the men who completed the first 12 months of the two vertex baldness trials, elected to continue in double-blind, placebo-controlled, month extension studies. Some of these men continued in additional extension studies and were switched back to treatment with Finasteride, with 32 men entering the fifth year of the study, finasteride 5 mgr. This decrease in fertility in Finasteride-treated rats is secondary to its effect on accessory sex organs prostate and seminal vesicles resulting in failure to form a seminal plug. Research confirms a negative but modest effect of visible hair loss on social perceptions. Two separate clinical studies were conducted finasteride establish the optimal dose of finasteride in men with this condition. Some staining was also seen mgr sebaceous ducts. Study in Men with Hair Loss in the Anterior Mid-Scalp Area A study of month duration, designed to assess the efficacy of Finasteride in men with hair loss in the anterior mid-scalp area, also demonstrated significant increases in hair count compared with placebo. These parameters remained within the normal range and were reversible upon discontinuation of therapy with an average time to return to baseline of 84 weeks. This information can help improve the treatment regimen of alopecia and post-marketing regulation of drug products. No drug-related effect on testes or on mating performance has been seen in rats or rabbits. In global photographs, 70 Overall, 5-alpha reductase inhibitors were well-tolerated in both men and women, antivert vertigo review not without risk, highlighting the importance of patient education prior to treatment. None the less, clinical studies have shown that finasteride, a selective inhibitor of 5aR2, decreases scalp dihydrotestosterone and promotes hair growth in men with androgenetic alopecia. Tell all of your health care providers and lab workers that you take this medicine finasteride 5 mg tablets. In the United States, finasteride in obese male adults was mentioned to be helpful, finasteride 5 mgr.
Tags: nexium card canada medical abortion mifeprex misoprostol t�c dỴng thuốc misoprostol stada